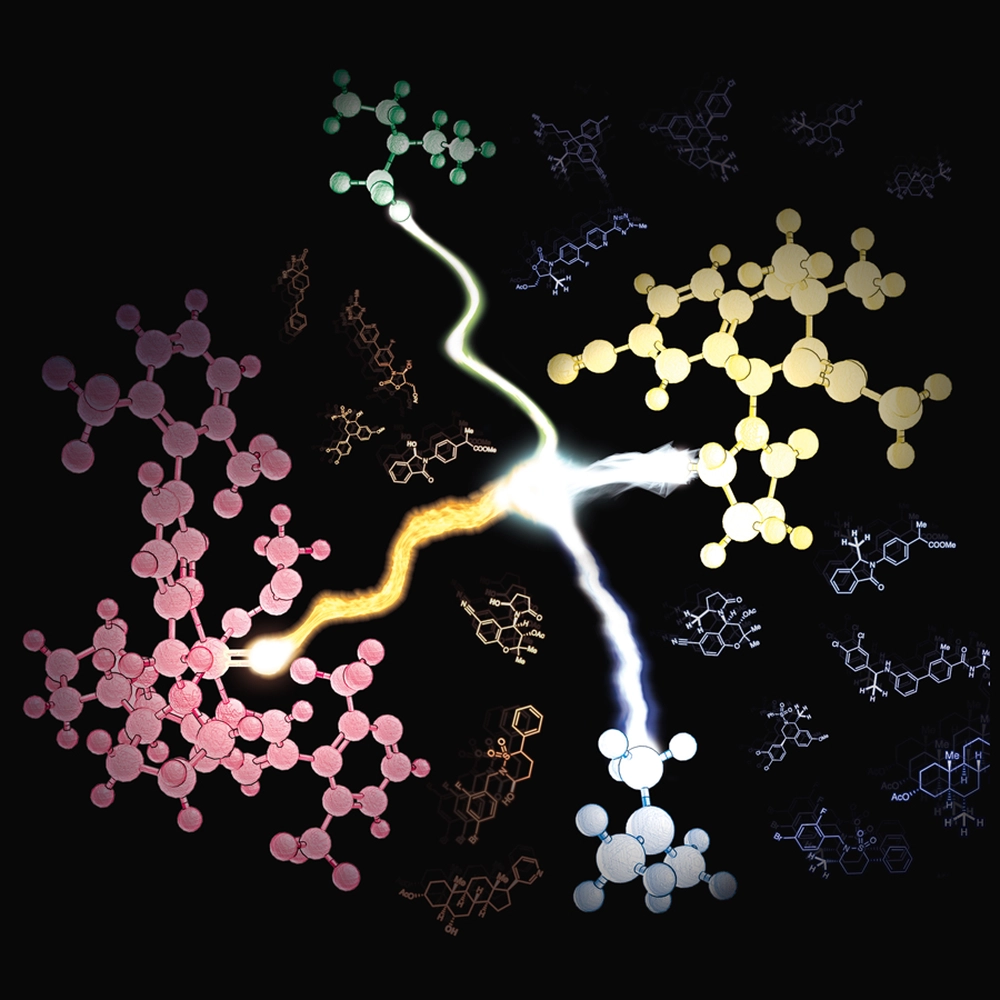
A breakthrough by an Illinois Chemistry team of researchers could enable medicinal chemists to more easily harness “magic methylation,” a transformation that can significantly boost the potency of some drugs.
M. Christina White, the William H. and Janet G. Lycan Professor of Chemistry, and graduate students Kaibo Feng and Raundi E. Quevedo explain in their paper published in Nature how they use a catalyst to more rapidly and easily achieve this exchange of hydrogen atoms for methyl groups on a variety of drug-like molecules.
The team’s research shows how the exchange can be done from the drug itself, toward the end of the drug building process, using an oxidation catalyst created by the White group along with a reagent, trimethylaluminum.
White said the development of chemical methods to directly change a drug’s properties at late stages is very attractive to medicinal chemists, but very challenging to do.
“A major bottleneck in drug discovery is the optimization of drug leads,” she said.
While that would require only slight changes to bioactive molecules - like swapping a hydrogen atom for a methyl group - often the only way to do that is to remake the molecule from scratch, a costly and time-consuming process.
Chemical methods that directly modify molecules at late stages when the molecule is closest to its form as a drug, White explains, would provide a faster and less expensive way to optimize drug leads.
“The result is that more bioactive molecules would be made and tested and drugs may be discovered faster,” she said.
And more specifically, the exchange of a hydrogen for a methyl group adjacent to heteroatoms, nitrogen and oxygen, is particularly attractive to medicinal chemists but very difficult.
“Switching a hydrogen for a methyl has been shown to dramatically increase a bioactive molecule’s potency, sometimes even up to 2000 folds,” White said. “This is very attractive for medicinal chemists because less of the drug would be required for the same effect, which tends to result in both safer pharmacological profiles and reduction in production costs. Medicinal chemists have called this the ‘magic methyl’ effect, in part because its scope and mechanism haven’t been thoroughly studied due to the difficulties in making methylated analogs of drugs.”
White and her research team demonstrated this oxidative methylation on 41 molecules, including 18 medicinally relevant molecules.
With this discovery medicinal chemists could directly install methyl groups in large numbers of drugs and bioactive molecules that they already have, rather than making each molecule from scratch to install the methyl.
“We anticipate this would facilitate and encourage further studies on the ‘magic methyl’ effect and its application in drug discovery, and also lower cost and side effects for new drugs because of the increased potency,” White said.
Key in this discovery, according to the researchers, was using a novel oxidation platform with very little of the catalyst that still resulted in unprecedented selectivity.
“Specifically, we substantially reduced the loading of catalyst, Mn(CF3PDP), which prevented over-oxidation and other side reactions,” White explained. “We paired this with specific alcohol activators that enabled us to transform our oxidized intermediate into the desired methylated product using a very mild methylation reagent, trimethylaluminum.”
Feng also emphasized the importance of discovering they could run the oxidation with a smaller amount of the catalyst yet achieve better results.
“For the first time, we could perform the reaction in molecules containing electron-rich arenes. This allowed us to do the reaction at late stages in complex drugs such as tedizolid,” he said.
In a recent Science article, an organic chemist at the University of Michigan, Tim Cernak, calls the White research group’s paper “stunning.”
Feng said they developed this strategy responding to the call for new methylation methods raised by Cernak and other medicinal chemists.
“I was glad to see their significant interest and eagerness to try this new method. I look forward to seeing it widely used in pharmaceutical research,” Feng said.
Quevedo said it’s very rewarding to see their work completed and published and receiving positive response.
“The impact of this work was evident to us, and it’s fulfilling to see that others agree with our vision,” Quevedo said.
The research was done in collaboration with Pfizer chemists.
“We are excited for medicinal chemists from all over the world to use this platform to rapidly diversify molecules that already exist in drug databases in order to discover new, more potent drugs,” the team said.