
Ananya Sen, Beckman Institute
Researchers from the University of Illinois at Urbana-Champaign have improved the technique of frontal polymerization, where a small amount of heat triggers a moving reaction wave that produces a polymeric material. The new method enables a wider range of materials with better control over their thermal and mechanical properties.
“This study demonstrates the Beckman Institute at its best,” said Jeff Moore, an Ikenberry Endowed Chair, a professor of chemistry, and the director of the Beckman Institute, where this project was completed. “It brought together two groups that have different perspectives on a problem but share a common goal.”
The paper, “Rapid Synthesis of Elastomers and Thermosets with Tunable Thermomechanical Properties,” was published in ACS Macro Letters and selected as ACS editors’ choice. Omar Alshangiti, a chemistry student who is an undergraduate researcher in the Moore Group, also made significant contribution to the study by investigating suitable monomer combinations, preparing most of the samples and measuring all the parameters of frontal polymerization process.
“Most of the previous research looked at stiffer materials. This paper is the first time frontal polymerization has been used to synthesize a rubbery material,” said Nancy Sottos, a Maybelle Leland Swanlund Chair and head of the Department of Materials Science and Engineering, who also leads the Autonomous Materials Systems Group at the Beckman Institute for Advanced Science and Technology. “The new technique allows us to have more control and makes materials that have good engineering properties in terms of strength and stiffness.”
The researchers used a mixture of two monomers, 1,5-cyclooctadiene and dicyclopentadiene, to create materials tailored for a wide range of applications.
“These materials are chemically similar to what is used in tires,” said Leon Dean, a graduate student in the Sottos Group, which is part of AMS. “Conventionally, the synthesis of rubbers requires an organic solvent, multiple steps, and a lot of energy, which is not environmentally friendly. Our solvent-free manufacturing method speeds up the process and reduces energy consumption.”
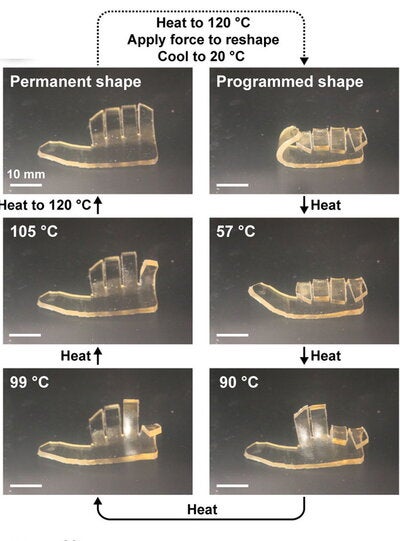
Using this technique, the researchers were able to make materials that demonstrate a shape memory polymer hand. The shape-memory effect occurs when a pre-deformed polymer is heated beyond its glass transition temperature, which is the point the polymer changes from a hard, glassy material to a soft, rubbery material. The sequential change in shape was enabled by the differences in glass transition temperature between each layer.
“We made a layered material in the shape of a hand, where each layer had different amounts of the two monomers and therefore different glass transition temperatures,” said Qiong Wu, a postdoctoral fellow in the Moore Group, which also is part of AMS. “When you heat the polymer above the highest glass transition temperature and then cool it, it forms a fist. As you raise the temperature again, the digits of the fist open sequentially.”
The researchers hope to further develop this technique by improving their control over the polymer properties. “Although we have demonstrated the tunability of several properties over a wide range, it remains a challenge to adjust each property individually,” Wu said.
“Scaling up the technique will also be a challenge,” Dean said. “Most of our work has been done on a lab scale. However, in larger scale manufacturing, there is a competition between bulk polymerization and frontal polymerization.”
This research was supported by the AFOSR Center for Excellence in Self-Healing, Regeneration and Structural Remodeling Award; the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering Award; and a National Science Foundation Graduate Research Fellowship.
Read more about the study “Rapid Synthesis of Elastomers and Thermosets with Tunable Thermomechanical Properties.”