By Tracy Crane, Department of Chemistry
To most, carbon monoxide is just a poisonous gas potentially lethal to humans, but scientific evidence has demonstrated in the last few decades its lesser-known potential as a therapeutic agent for a host of human diseases and conditions.
The challenge has been figuring out how to safely deliver it to the body, and that’s why chemistry researchers at the University of Illinois at Urbana-Champaign are excited about their discovery of a new mechanophore, a force sensitive molecule, that can release controlled amounts of CO when triggered by mechanical force.
William Neary, postdoctoral researcher, Yunyan Sun, graduate researcher, and Jeffrey Moore, professor in the Department of Chemistry and director of the Beckman Institute for Advanced Science and Technology, explain in a study published in The Journal of the American Chemical Society their discovery of a mechanophore motif, called norborn-2-en-7-one, or NEO, that releases controlled amounts of carbon monoxide when installed in a polymer chain and stimulated with ultrasonication and even grinding or shearing.
The researchers believe this is the first example of a CO release triggered by mechanical force. But that’s not all they discovered. After the CO release, the resulting material emits a unique fluorescence, named aggregation-induced emission (AIE), allowing the researchers to visualize the amount of carbon monoxide released during the experiment. By adding water to promote the aggregation following the release of CO, the researchers could clearly observe a bright cyan emission under ultraviolet light after applying mechanical force.
This is both the first reported bifunctional mechanophore – release of CO and AIE – and the first account of AIE being turned on in a polymer upon mechanochemical activation, making the NEO important for both fundamental research and potential therapeutic applications.
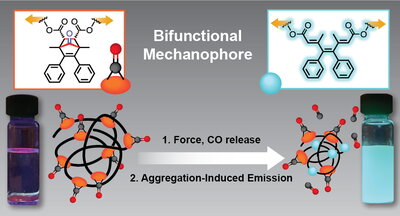
“Although the molecular motif we employed in this report was known to release CO under other stimuli such as heat and UV light via cheletropic extrusion, a classical undergraduate textbook reaction, we hypothesized that CO is released via a completely different mechanism by simply applying force to this molecule from opposite directions,” Neary explained.
The researchers believe NEO has a lot of potential as a CO delivery method. As they explain in their report, “polymer mechanochemistry is a potentially useful platform for the controlled release of small molecules,” like CO, for therapeutic applications, because ultrasound can “penetrate deep within biological tissues to achieve mechanochemical transformations noninvasively and with precise spatial and temporal control.”
This odorless, tasteless, noxious gas that kills hundreds and hospitalizes thousands in the U.S. each year when inhaled in large quantities from faulty furnaces or vehicle exhaust can actually be beneficial to humans in small quantities.
The human body naturally produces CO in small amounts intracellularly via the heme oxygenase enzyme, which plays a role in counteracting vascular and inflammatory disorders. Through this pathway, studies have shown that CO could help combat a multitude of human conditions and disease and is now being developed as a therapeutic agent due to its cytoprotective, antibacterial, anti-inflammatory and anticancer effects.
“CO is important,” Neary said, “but deploying it to humans is a much more challenging task.”
Administering CO through inhalators proved difficult and safer delivery methods are needed. Recent advances in CO releasing molecules (CO-RMs), which are compounds that can carry and release specific amounts of carbon monoxide within cellular systems, have overcome some of the challenges and potential dangers of CO inhalation, Neary explained.
“But advances and alternative delivery modes, such as polymer mechanochemistry, could serve as the next potential breakthrough,” Neary said.
Sun said the pulsed solution ultrasonication technique they used to stimulate the mechanophore is common in the field, but in their study, they also demonstrated more simple ways to trigger the reaction.
“You can take a pestle and smash it or grind it to transduce force to the polymer to trigger the reaction to release CO and see the cyan emission, all in a pretty short amount of time, like several minutes,” Sun explained.
In the quest to find new uses for mechanoresponsive materials, the discovery of chemistries capable of small molecule release are highly desirable, the researchers explain in their study. Neary and Sun were initially searching for a mechanophore that would undergo a cheletropic reaction but eventually discovered this promising molecular motif.
“We found this motif that gave us almost everything that we wanted, pretty simple synthesis, it was scalable and it was stable and it was pretty efficient for the release of carbon monoxide,” Neary said. “Benefiting from this new reaction pathway, we were able to achieve upwards of 154 CO molecules released from a single polymer chain.”
Sun said that one of the challenges in terms of using mechanical force to release small molecules is the loading ratio.
“In a lot of the examples, the mechanophores are scissile, meaning once they are activated the entire chain will break, so they can only release one single small molecule in one polymer chain, but because our mechanophore is nonscissile -- which there are only a few examples of right now -- we could achieve more than 100 CO molecules released in one polymer chain,” Sun explained. “This is really one of the advantages of our system.”
That advantage was also one of the challenges in the design process, Neary said.
“There’s not a lot of precedent of small molecule-releasing mechanophores that release a large amount of material, and that was something that we wanted to do, so that was definitely a challenge synthetically in the design,” he said.
Neary said they are really excited about this initial report and what the future may hold. The research team believes the NEO mechanophore could lead to exciting biological and biomedical opportunities as well as serve as a guide for the development of novel force-responsive AIE luminogens for damage detection and bioimaging applications.
Sun said the hope is to combine this newly designed mechanophore with high intensity focused ultrasound to expand this research to in vitro studies and in vivo studies with mice first and eventually the human body.
“One of the directions we are pursuing right now is to try to kill cancer cells in the tumor,” Sun said.
Moore credits his research group members, Neary and Sun, with the idea that led to this research.
“When I was a postdoc with Bob Grubbs, he said, ‘There will come a time when the best ideas emerge from group members. Just get out of their way and let them do their thing.’ He was right. This is a fine example of that advice,” Moore said.
This research is financially supported by the Center for the Chemistry of Molecularly Optimized Networks (MONET), a National Science Foundation (NSF) Center for Chemical Innovation.
Editor’s note:
The paper associated with this work can be accessed at https://pubs.acs.org/doi/10.1021/jacs.1c12108.
Contact Jeffrey Moore at jsmoore@illinois.edu