
CHAMPAIGN, Ill. – An Illinois research team has discovered a way to produce a special class of molecule that could open the door for new drugs to treat currently untreatable diseases.
Open the household medicine cabinet and you will likely find organic derivatives of ammonia, called amines. They are one of the most prevalent structures found in medicines today. More than 40 percent of drugs and drug candidates contain amines, and 60 percent of those amines are tertiary, so named for the three carbons that are bonded to a nitrogen.
Tertiary amines are found in some of the most impactful human medicines, including antibiotics, breast cancer and leukemia drugs, opioid pain medications, antihistamines, blood thinners, HIV treatments, antimigraine medications and more. They increase a drug’s solubility and can trigger its key biological functions.
Despite the prevalence of this special class of molecules in medicines today, much of the functional potential of tertiary amines likely remains untapped.
That’s because the traditional process of making them requires specific, controlled conditions that inherently limit the discovery of new tertiary amines, which could potentially treat a wide range of currently untreatable diseases.
Now, an Illinois research team led by Lycan Professor of Chemistry M. Christina White and graduate students Siraj Ali, Brenna Budaitis, and Devon Fontaine have discovered a new chemical reaction, a carbon-hydrogen amination cross-coupling reaction, that creates a faster, simpler way of making tertiary amines without the inherent limitations of classic methods. The researchers believe this could also be used to discover new reactions with nitrogen.

This new reaction in the chemist’s toolbox transforms the traditional tertiary amine building process – with its classic chemical reactions that require highly-specialized conditions specific to each molecule -- into a procedure that can be carried out in general conditions open to air and moisture with the potential for automation.
As the researchers describe in their paper in Science (DOI:10.1126/science.abn8382), this new procedure uses a metal catalyst discovered by their group (Ma-WhiteSOX/palladium) and two building blocks— abundant hydrocarbons (olefins containing adjacent C—H bond) and secondary amines— to generate a variety of tertiary amines.
This has the potential, White explained, for chemists to take a lot of different secondary amines and couple them to a lot of different olefins, both of which you can either buy or easily make.
“And these are stable starting materials. You could have them in individual containers, mix and match them, and using our catalyst make many different combinations of tertiary amines,” White said. “The flexibility of this reaction makes the discovery process for tertiary amine drugs easier.”
The difference between classical reactions and this new reaction for making tertiary amines is like the difference between picking a specialty sandwich from a menu versus creating your own sandwich from a diverse set of ingredients – you have a lot more flexibility in terms of choices.
This highly flexible system for making tertiary amines is also very practical.
“You could, in principle, run it on your stove top,” White explains. “You don’t need to handle it with a lot of precautions, you can run it open to air and you don’t have to exclude water. You just need your starting materials, the palladium/SOX catalyst and a little heat. It should work just the way we are doing it in the lab.”
White explained that when a pharmaceutical company wants to make tertiary amines, they often have to use specialized procedures, but this reaction allows you to take two simple, often commercial, starting materials and put them together using the same procedure.
“Because the conditions are so simple and work for so many different amines and olefins there is great potential to adopt this reaction for automation,” White said.
The major challenge the team overcame in this discovery was solving a long-standing problem in C—H functionalization chemistry: replacing a hydrogen atom on a molecule’s carbon framework with a basic, secondary amine to directly make tertiary amines.
Metal catalysts prefer interacting with basic amines rather than the C—H bonds in the olefin. The team hypothesized that amine salts (amine-BF3 salts that are easy to use and store) can prevent this interaction with the catalyst.
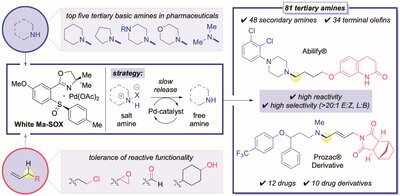
Like a dam modulating the flow of water, the team’s palladium/SOX catalyst regulates the slow release of amines from the salts as well as mediates coupling the secondary amine and hydrocarbon to form the tertiary amine product.
Showcasing the power of this new chemical reaction, the researchers made 81 tertiary amines in their study, coupling a wide range of complex, medicinally relevant secondary amines to many complex olefins containing reactive functionality. This includes functionality that is reactive with secondary amines in the traditional tertiary amine manufacturing processes.
Further demonstrating the potential to discover new medicines, the research team also applied this new reaction to the efficient syntheses of 12 existing drug compounds, including Abilify, an anti-psychotic medication, Naftin, an anti-fungal, as well as 11 complex drug derivatives, including the anti-depressants, Paxil and Prozac, and the blood-thinner, Plavix.
In addition to this reaction being used in the pharmaceutical industry as a platform to expedite the discovery of new tertiary amine drugs, the researchers also believe that their catalyst-controlled slow-release strategy could be used by other researchers to discover many additional new reactions with nitrogen.
— Tracy Crane, Communications Specialist, Department of Chemistry
This research in the news:
Editor’s notes:
To reach M. Christina White, email mcwhite7@illinois.edu.
To reach Siraj Ali, email szali2@illinois.edu.
To reach Brenna Budaitis, email budaiti2@illinois.edu.
To reach Devon Fontaine, email dff2@illinois.edu.
The paper “Allylic C–H amination cross-coupling furnishes tertiary amines by electrophilic metal catalysis” is available online. DOI: 10.1126/science.abn8382