
When a human consumes a pharmaceutical drug, enzymes in the liver break down the substance into metabolites that are water soluble, so the body can more easily excrete them. In some cases, the resulting metabolites may have potent effects that can be good or bad.
Medicinal chemists must test drug candidates for these potential effects, and the only way is with large quantities of the metabolites that they make “from scratch” in a lengthy sequence of chemical reactions, requiring a lot of time, labor, and materials.
Now, a research team at the University of Illinois Urbana-Champaign that was led by M. Christina White, William H. and Janet G. Lycan Professor of Chemistry, and included graduate students Rachel Chambers and Jake Weaver and visiting scholar Jinho Kim, collaborated with scientists at Merck & Co. to develop a rapid and efficient method of making large quantities of metabolites directly from a drug or drug precursors via carbon-hydrogen oxidation catalysis.
The critical component of the method is the White-Gormisky-Zhao catalyst [Mn(CF3-PDP)] that can mimic the natural function of the CYP450 liver enzyme in oxidizing drugs and breaking them down to metabolites.
Hear the research team explain the science behind their discovery in this video presentation.
Their study, “A preparative small-molecule mimic of liver CYP450 enzymes in the aliphatic C—H oxidation of carbocyclic N-heterocycles,” was recently published in PNAS and details this catalyst’s ability to oxidize drugs and drug-like molecules like CYP450 liver enzymes, furnishing metabolites on a large scale in just one to two steps from the drug or drug precursor. The paper is available online: https://doi.org/10.1073/pnas.2300315120.
In the study, the researchers demonstrate this process on large scales in pharmaceutical compounds like the antipsychotic blonanserin, COX-2 inhibitors, and the fungicide penconazole.
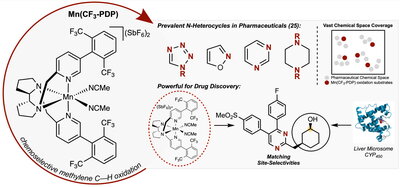
White said the White-Gormisky-Zhao catalyst is like a “P450 mimic in a bottle.”
Chambers said this work has important implications for the study of metabolism, because Carbon—Hydrogen oxidation is one of the key steps performed by the human body when it eliminates drugs in the metabolic process.
“Understanding and being able to mimic these processes with chemical reactions could lead to the development of new drugs or the modification of existing ones to improve their efficacy and reduce side effects,” Chambers said.
Researchers said the key to this discovery is the substantial expansion of their catalyst’s ability to introduce oxygen into a broad range of heterocycle-containing molecules, which are very common structures in man-made drugs. Nitrogen containing heterocycles are present in 59 percent of FDA approved pharmaceuticals, according to the study.
The researchers report that their catalyst works in the presence of 25 nitrogen heterocycles, including 14 of the 27 most frequent N-heterocycles in man-made drugs. Researchers said the expansion of scope was guided and quantitatively evaluated by a chemoinformatics analysis that supports their catalyst’s potential for significantly expanding the pharmaceutical chemical space now accessible with small molecule Carbon—Hydrogen oxidation catalysis.
Weaver said prior to this work, oxidizing C-H bonds in the presence of many types of nitrogen heterocycles had rarely been demonstrated.
“Because of this, many classes of pharmaceuticals would have been considered ‘untouchable’ for a C-H oxidation reaction. Our reaction opens the door for many new types of nitrogen-containing drugs to be compatible with C-H oxidation,” Weaver said.
An emerging trend in small molecule pharmaceuticals generally comprised of N-heterocycles is the incorporation of aliphatic fragments that improve their potency and solubility. But this makes it more challenging to study metabolite effects. The reason, White said, is making drug metabolites where the oxidation happens on the aliphatic fragments is difficult due to the propensity of most reactions to oxidize the nitrogen heterocycle.
In general, Weaver explained, nitrogen-containing molecules have low tolerance for C-H oxidation catalysts. To address that problem, the team used their catalyst in combination with an HBF4 protonation strategy that deactivates the nitrogen from being oxidized while allowing the desired remote aliphatic Carbon—Hydrogen oxidation to occur.
White said that strategy has shown unprecedented selectivity for oxidizing the aliphatic fragments of drugs while leaving the nitrogen heterocycles untouched.
Financial support for this work was provided by the National Institute of General Medical Sciences (NIGMS) Maximizing Investigators’ Research Award, a grant from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., and the Molecule Maker Lab Institute (An AI Research Institutes program supported by the National Science Foundation, NSF under award No. 2019897) for support for chemoinformatic analysis.
The research paper is available at https://doi.org/10.1073/pnas.2300315120.
Editor’s note:
You can reach M.C. White at mcwhite7@illinois.edu.
This research in the news:
— Story by Tracy Crane, Department of Chemistry