
When Nobel laureate Omar M. Yaghi (PhD, '90) was recently asked how he made the choice as a young scientist to pursue the idea of creating porous materials, he talked about two people in his life — his father and Walter Klemperer, his PhD advisor at the University of Illinois Urbana-Champaign. In different ways, both inspired him to follow his own path.
"I had two people in my life who were very significant. My father, who constantly urged me to think differently and go in my own direction and not to follow other’s footsteps; to create something completely new and not to be a follower," Yaghi said. "And somehow I was fortunate to join the group of Walter Klemperer at the University of Illinois as a graduate student, and there again, the same lesson, except now we are talking about molecules. He (Klemperer) says, well, I want you to make something that no one has made before and challenged me to go beyond what is out there."
Yaghi added that Klemperer taught him how to be a rigorous scientist.

"So, if it wasn’t novel, it wasn’t worth doing with these two characters who were in my life," Yaghi said. "So, I would say that was quite important but then you have to really understand that it’s very risky. It requires hard work. It’s full of failure as all great things are and you just have to sustain and believe in the power of the experiment. That would be my advice to the young people is believe in the power of the experiment. When you do the experiment, you open the door to make big discoveries. If you don’t do the experiment, then nothing is going to happen."
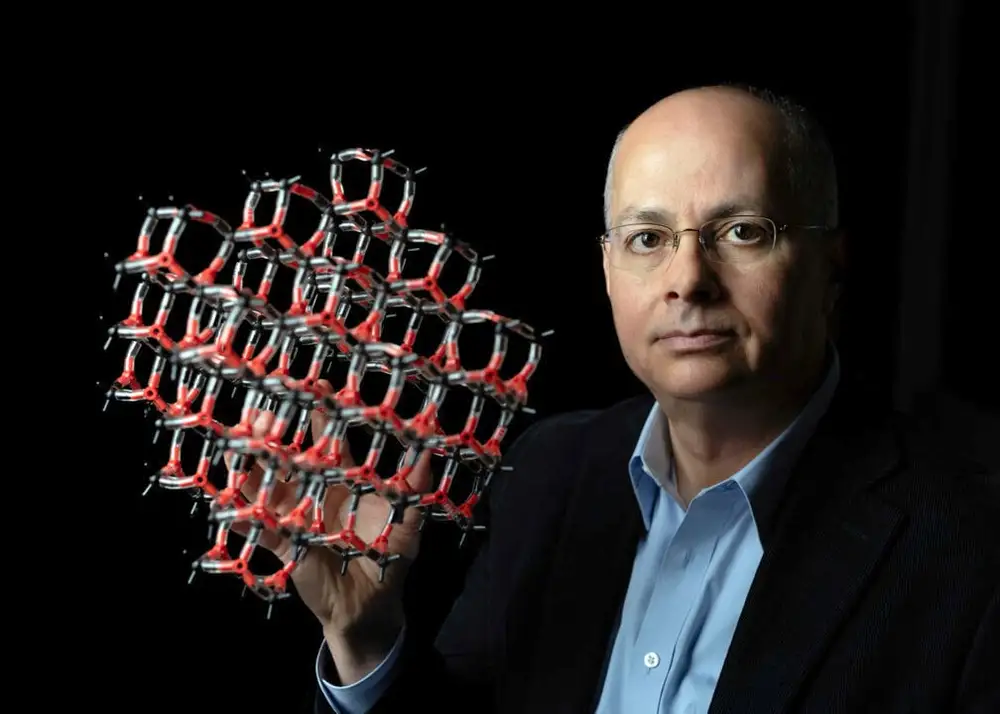
Yaghi, 60, continued to take risks, work hard, persevere through failure, and believe in the power of the experiment as he pursued his work encompassing the synthesis, structure and properties of inorganic and organic compounds and the design and construction of new crystalline materials. He is widely known for the discovery and for pioneering the development of several extensive classes of new materials: Metal-Organic Frameworks (MOFs), Covalent Organic Frameworks (COFs), and Zeolitic Imidazolate Frameworks (ZIFs).
And on Oct. 8, his work was recognized with the Nobel Prize in Chemistry, which he shares with two other scientists. Yaghi is the 12th Nobel Prize winner among graduates, postdocs, or faculty in the Department of Chemistry at Illinois.
Yaghi shares the prize with Susumu Kitagawa, Kyoto University, Japan, and Richard Robson, University of Melbourne, Australia, “for the development of metal–organic frameworks”. The Nobel Prize laureates created molecular constructions with large spaces through which gases and other chemicals can flow. These constructions, metal–organic frameworks, can be used to harvest water from desert air, capture carbon dioxide, store toxic gases or catalyze chemical reactions.
Yaghi was born Feb. 9, 1965, in Amman, Jordan. He received his B.S. from State University of New York at Albany (1985) and his Ph.D. in Inorganic Chemistry from Illinois in 1990 before working as an NSF Postdoctoral Fellow at Harvard University (1990-92). He started his independent career as an assistant professor in 1992 at Arizona State University, moved to University of Michigan at Ann Arbor as Robert W. Parry Professor of Chemistry in 1999, and then UCLA in 2006.
Since 2012 he has been the James and Neeltje Tretter Chair Professor of Chemistry at University of California, Berkeley, and a Senior Faculty Scientist at Lawrence Berkeley National Laboratory. He is the Founding Director of the Berkeley Global Science Institute, and the Co-Director of the Kavli Energy NanoSciences Institute, and the California Research Alliance by BASF.
Yaghi calls his field “reticular chemistry,” which he defines as “stitching molecular building blocks into crystalline, extended structures by strong bonds.”
In a UC Berkeley article, Yagh said the idea came to him around the time he obtained his Ph.D. from the University of Illinois at Urbana-Champaign in 1990.
Here is an excerpt from that article:
There, he worked with chemist Walter Klemperer, who Yaghi said taught him not only “how to do rigorous science and the value of evidence in making your data analysis and conclusion,” but also “how to become a scientist that can break new ground in science, not somebody who follows other people’s discoveries.”
Yaghi had been working on the synthesis of complex molecules by building up larger molecules and then tearing them apart to get the desired structure, but he said the result was “unsatisfactory.” Just as polymers are strings of identical molecules, he envisioned two-dimensional or even 3D polymers, like crystals, constructed from the same molecular building blocks.
“There was no rationality in how you made these materials. There was no design, no intellectual rules or guidance for making them,” Yaghi said. ”So I was fixated, as an assistant professor at Arizona State University in Tempe, on building materials using a building block approach so that I could rationally put these things together.”
After unsuccessfully searching for totally organic crystalline structures, he tried hybrids of inorganic metals and organic molecules. Earlier metal-organic compounds — coordination polymers, as they were called — had been hard to use as porous structures because they were frail, but Yaghi and his students at Arizona State tried a different approach. They created an inorganic cluster of two metal atoms — a dimetal carboxylase — and linked it with a standard organic molecule that could be ordered from a supply house. After many trials, Yaghi’s team was able to create a stable, crystal-like structure with clusters of two metal atoms at the corners of a cube and the ligands between. He soon achieved success with clusters of four metal atoms and showed that the structures were robust, stable against degradation and highly porous.
“That basically was the spark that ignited the field,” he said. “After that, anybody could take an inorganic cluster, link it with an organic ligand and make a porous crystal. You can functionalize the pores, do hydrogen storage, CO2 capture, you can now capture water. And on top of all of that, you have thousands of inorganic building blocks that could be used and millions of organic units that could be used, and the combination would produce an infinite, truly infinite variety of structures that can not only be imagined, but can actually be made in the lab.”
One of the most important advantages of MOFs is that they have an enormous surface area within their pores: as much as 10,000 square meters per gram of MOF, the equivalent of two football fields. The huge internal surface area allows a large volume of gas to stick, or adsorb. In addition, the metal and organic components of an MOF can be adjusted to select the type of gas adsorbed and how tightly it sticks.
“The metal clusters are at the corners of a scaffolding, like they put around a building,” Yaghi said. “At the intersection, people had put one metal ion. The new ones that we invented had clusters of metal ions that were large and allowed you to have flexibility on how they are linked. And above all else, they were not flimsy, they were not unsteady, like the ones made from single metal ions. The strong bonds between the metal clusters and charged organic linkers basically make the framework steady and robust.”
With MOFs, Yaghi essentially combined the fields of organic chemistry — the chemistry of carbon compounds — and inorganic chemistry, which deals with everything else, and extended those fields to 2D and 3D materials. These MOFs were highly customizable. By adjusting the two building blocks, the size and chemical environment of the pores inside the MOF crystals could be tailored for a given application.
By adding enzymes to the interior of the pores, MOFs can also be tooled to catalyze reactions, such as converting methane to methanol or breaking water into hydrogen and oxygen, providing clean energy.
Yaghi’s initial reports on MOFs were met with skepticism and sometimes derision, he said. But he had the support of a few chemists — key to a young assistant professor just starting his career — that kept him focused on the new field. He continued to work on MOFs after moving to the University of Michigan in 1999, to UCLA in 2007 and to UC Berkeley in 2012.
The field has continued to grow, Yaghi said. The number of articles on MOFs in scientific journals balloons each year and has yet to plateau. Dozens of companies in the U.S. are pursuing research on MOFs, with applications ranging from safer ways to store dangerous chemicals to ways to create better catalysts. He is among the top five most highly cited chemists worldwide.
Yaghi was born in Amman, Jordan, in 1965 to parents who were refugees from Palestine. His father raised cattle and owned a butcher shop in Amman.
At the age of 15, he was told by his father that he must go to the U.S. to study and, within the year before he graduated from high school, he had obtained a visa and settled alone, in Troy, New York, to pursue his college education.
With a poor grasp of English, Yaghi took courses in English, math and science at Hudson Valley Community College in Troy before transferring to the State University of New York at Albany in 1983.
“I was in love with chemistry from the very beginning,” Yaghi said. “And when I moved to Albany, I immediately got into research. I was doing three different projects with three different professors at the same time: a physical organic project with one professor, a biophysical project with another and a theory project with a third professor. I really loved the lab. I disliked class, but I loved the lab.”
Nobel prize announcement
In the Oct. 8 Nobel prize announcement, Heiner Linke, Chair of the Nobel Committee for Chemistry, said metal–organic frameworks have enormous potential, bringing previously unforeseen opportunities for custom-made materials with new functions.
It all started in 1989, when Richard Robson tested utilizing the inherent properties of atoms in a new way. He combined positively charged copper ions with a four-armed molecule; this had a chemical group that was attracted to copper ions at the end of each arm.
When they were combined, they bonded to form a well-ordered, spacious crystal. It was like a diamond filled with innumerable cavities.
Robson immediately recognized the potential of his molecular construction, but it was unstable and collapsed easily. However, Susumu Kitagawa and Omar Yaghi provided this building method with a firm foundation; between 1992 and 2003 they made, separately, a series of revolutionary discoveries. Kitagawa showed that gases can flow in and out of the constructions and predicted that MOFs could be made flexible. Yaghi created a very stable MOF and showed that it can be modified using rational design, giving it new and desirable properties.
Following the laureates’ groundbreaking discoveries, chemists have built tens of thousands of different MOFs. Some of these may contribute to solving some of humankind’s greatest challenges, with applications that include separating PFAS from water, breaking down traces of pharmaceuticals in the environment, capturing carbon dioxide or harvesting water from desert air.