A team of chemists led by M. Christina White, William H. and Janet G. Lycan Professor of Chemistry at the University of Illinois Urbana-Champaign, has unveiled a breakthrough in selective oxidation chemistry that could significantly advance drug development with natural products.
In a recently published paper in Nature, they reveal a novel catalytic system that enables the first chemoselective oxidation of strong methylene bonds in compounds containing α, β-unsaturated carbonyls, moieties that are common in biologically active natural products and their derivatives. Examples are fukinone, which has anti-inflammatory properties, finasteride, a molecule prescribed for hair loss and prostate enlargement, and glychyrrhizin, which gives licorice its sweet taste.
“Although natural products are a rich source for medicines, they are less frequently explored by pharmaceutical companies because their complexity makes modifications too difficult,” White said.
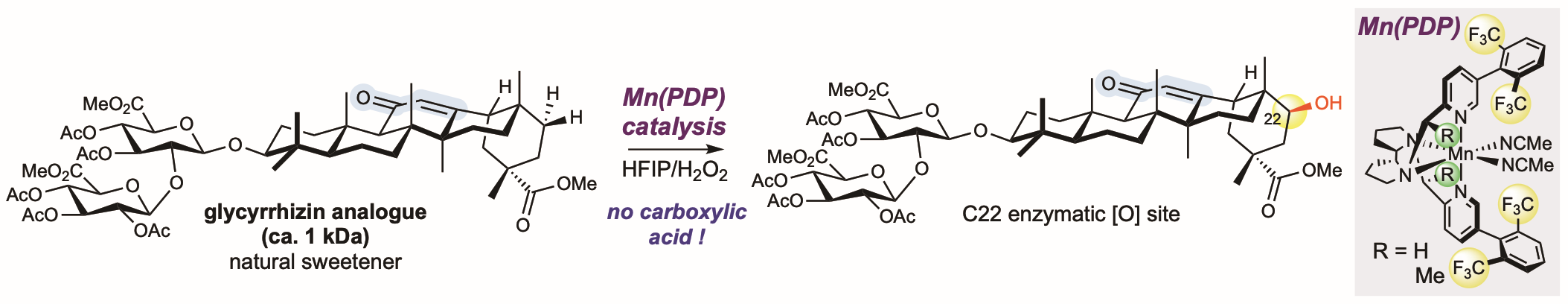
Chiyoung Ahn, the lead graduate student author on the study, said using the new PDP-catalyzed reaction the team was able to selectively oxidize glycyrrhizin, a complex diglucuronide pentacyclic triterpenoid, that has the molecular weight of a small protein (ca. 1 kDa).
Alex Gomez, another graduate student co-author, said the method was applied to a streamlined and diversifiable total synthesis of the antibiotic platencin, providing access to the natural product as well as two novel structural analogs that would otherwise require de novo syntheses.
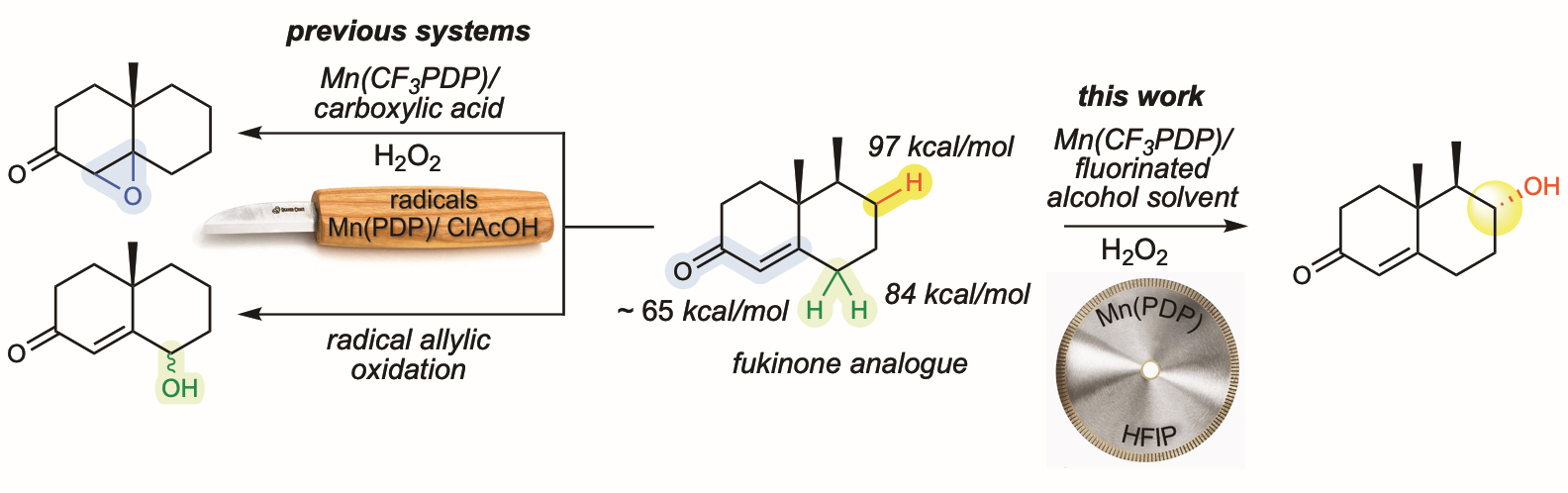
According to the researchers, traditional oxidation methods would destroy the medicinally important α, β-carbonyl functionality that is responsible for the biological activities of these natural products. The reason for this is that both the electron deficient allylic C—H bond and the pi-olefin bond of α, β-unsaturated carbonyls are weak and easily oxidized with reagents that cleave strong C—H bonds.
The researchers wondered if a chemical reaction using a commercial catalyst – Mn(CF3PDP) – developed in their lab- could enable late-stage oxidations in these natural products because their catalyst does not generally cleave electron deficient C—H bonds, even when they are weak. They discovered that while it did not cleave the allylic bond, it did oxidize the very weak and easily accessible pi-bond of the olefin to an epoxide.
The team’s solution to this problem was to change the active Mn(CF3PDP) oxidant to one that works by a different mechanism. Previously, their group had shown that adding carboxylic acids (key components in vinegar) turns on the ability of metal PDPs to perform both C—H oxidation and epoxidation. The carboxylic acid additive works with hydrogen peroxide at the metal to make the active oxidant. In this work, they discovered that removing the carboxylic acid and adding an electron deficient alcohol solvent made C—H oxidation 39 times faster than the olefin oxidation. The researchers draw an analogy of their new Mn(CF3PDP) oxidant – that cuts strong C—H bonds but leaves weaker allylic and pi-bonds intact – to a diamond blade. Unlike a carving knife, a diamond blade cuts through hard stone but leaves soft surfaces (like skin) intact because of its mechanism of action that grinds rather than slices.
The team demonstrated the method’s broad applicability across 45 structurally diverse molecules containing α,β-unsaturated carbonyl groups. In all complex molecules examined, the new catalyst system outperformed existing methods.
Editor’s notes:
This research was supported by funding from the National Institutes of Health.
The published paper is in Nature in Advanced Article Preview at https://doi.org/10.1038/s41586-025-09742-0.
For more information, contact:
M. Christina White at mcwhite7@illinois.edu