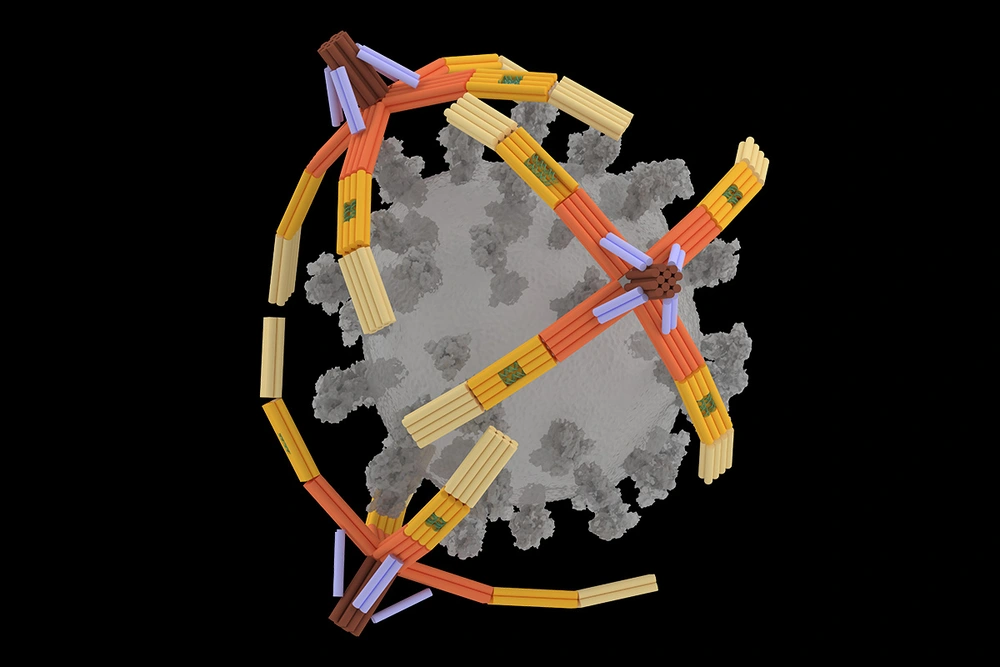
A tiny, four-fingered “hand” folded from a single piece of DNA can pick up the virus that causes COVID-19 for highly sensitive rapid detection and can even block viral particles from entering cells to infect them, University of Illinois Urbana-Champaign researchers report. Dubbed the NanoGripper, the nanorobotic hand also could be programmed to interact with other viruses or to recognize cell surface markers for targeted drug delivery, such as for cancer treatment.
Led by Xing Wang, a professor of bioengineering and of chemistry at the U. of I., the researchers describe their advance in the journal Science Robotics.
Inspired by the gripping power of the human hand and bird claws, the researchers designed the NanoGripper with four bendable fingers and a palm, all in one nanostructure folded from a single piece of DNA. Each finger has three joints, like a human finger, and the angle and degree of bending are determined by the design on the DNA scaffold.
“We wanted to make a soft material, nanoscale robot with grabbing functions that never have been seen before, to interact with cells, viruses and other molecules for biomedical applications,” Wang said. “We are using DNA for its structural properties. It is strong, flexible and programmable. Yet even in the DNA origami field, this is novel in terms of the design principle. We fold one long strand of DNA back and forth to make all of the elements, both the static and moving pieces, in one step.”
The fingers contain regions called DNA aptamers that are specially programmed to bind to molecular targets — the spike protein of the virus that causes COVID-19, for this first application — and trigger the fingers to bend to wrap around the target. On the opposite side, where the wrist would be, the NanoGripper can attach to a surface or other larger complex for biomedical applications such as sensing or drug delivery.
To create a sensor to detect the COVID-19 virus, Wang’s team partnered with a group led by Illinois electrical and computer engineering professor Brian Cunningham, who specializes in biosensing. They coupled the NanoGripper with a photonic crystal sensor platform to create a rapid, 30-minute COVID-19 test matching the sensitivity of the gold-standard qPCR molecular tests used by hospitals, which are more accurate than at-home tests but take much longer.
“Our test is very fast and simple since we detect the intact virus directly,” Cunningham said. “When the virus is held in the NanoGripper’s hand, a fluorescent molecule is triggered to release light when illuminated by an LED or laser. When a large number of fluorescent molecules are concentrated upon a single virus, it becomes bright enough in our detection system to count each virus individually.”
In addition to diagnostics, the NanoGripper could have applications in preventive medicine by blocking viruses from entering and infecting cells, Wang said. The researchers found that when NanoGrippers were added to cell cultures that were then exposed to COVID-19, multiple grippers would wrap around the outside of the viruses. This blocked the viral spike proteins from interacting with receptors on the cells’ surface, preventing infection.
“It would be very difficult to apply it after a person is infected, but there’s a way we could use it as a preventive therapeutic,” Wang said. “We could make an anti-viral nasal spray compound. The nose is the hot spot for respiratory viruses, like COVID or influenza. A nasal spray with the NanoGripper could prevent inhaled viruses from interacting with the cells in the nose.”
An artistic rendering of the NanoGripper’s applications. Sites on the gripper’s fingers recognize the spike protein of a virus, inset, and trigger fluorescent tags to emit light. When coupled with a sensor, individual viruses can be detected for a rapid COVID test, foreground. Alternately, the NanoGrippers can block viruses from entering cells by wrapping around the spike proteins, top right.
The NanoGripper could easily be engineered to target other viruses, such as influenza, HIV or hepatitis B, Wang said. In addition, Wang envisions using the NaoGripper for targeted drug delivery. For example, the fingers could be programmed to identify specific cancer markers, and grippers could carry cancer-fighting treatments directly to the target cells.
“This approach has bigger potential than the few examples we demonstrated in this work,” Wang said. “There are some adjustments we would have to make with the 3D structure, the stability and the targeting aptamers or nanobodies, but we’ve developed several techniques to do this in the lab. Of course it would require a lot of testing, but the potential applications for cancer treatment and the sensitivity achieved for diagnostic applications showcase the power of soft nanorobotics.”
The National Institutes of Health and the National Science Foundation supported this work. Wang and Cunningham are affiliated with the Carl R. Woese Institute for Genomic Biology and the Holonyak Micro and Nanotechnology Lab at the U. of I.
Editor’s note:
To reach Xing Wang, email xingw@illinois.edu.
The paper “Bioinspired designer DNA NanoGripper for virus sensing and potential inhibition” is available online. DOI: 10.1126/scirobotics.adi2084
This work was supported in part by NIH grants R21EB031310, R44DE030852 and R21AI166898.
In the news:
University of Illinois researchers develop DNA NanoGripper to detect, inhibit viruses - CBS Chicago
Scientists Made a Tiny 'Hand' to Snatch Viruses From Your Body
